Here are the facts:
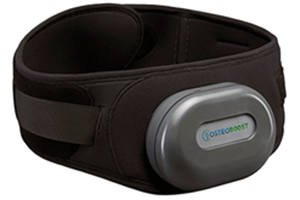
On January 18, 2024, the FDA provided clearance for a new medical product called Osteoboost™, a wearable vibrating belt intended to reduce bone loss and encourage bone growth in the hips and spine. Inspired by technology hoping to prevent bone loss in astronauts, the Osteoboost™ belt was developed by a California-based startup called Bone Health Technologies (1).
As stated by the company’s CEO Laura Yecies: “This groundbreaking decision represents the first non-pharmacological therapy approved to treat this widespread and serious condition”.
The FDA approval was based on the results of a double-blind study (2) funded by the National Institutes of Health, that included 126 post-menopausal women with low bone density, commonly referred to as osteopenia. Sixty-four subjects used the actual belt and sixty-two were issued a “sham” which emitted sound but did not vibrate. All subjects used the belt five times/week for one year. The Osteoboost™ wearers who used the device at least three times per week lost about 0.5% bone strength, while the control group lost about 2.8%, as analyzed by CT scan. This is a statistically significant result. No adverse side effects were reported.
Once it becomes available (sometime this year), the instructions for use will be to wear the belt for 30 minutes every day or at least five times a week for it to fully take effect. It reportedly delivers a gentle vibration, and the user is encouraged to wear it during routine, everyday activities, such as walking. It features an accelerometer and a pressure sensor which gauges the amount of vibration reaching the bones, (dependent on the amount of soft tissue and clothing between the device and the targeted bones), and will automatically adjust for optimal effectiveness.
A prescription will be required from your doctor. As far as cost is concerned, the pricing will vary depending on the insurer and co-pays but is expected to be under $1000.00.
Here is my opinion:
Osteoboost™ shows promise in slowing the loss of bone density, but keep in mind, the clearance at this point is for people with osteopenia. These results are hopeful and intriguing, but as with any scientific report, additional independent studies must be done to corroborate the original findings.
My biggest concern is the disclosure from the researchers of a clear conflict of interest. Of the nine authors, six are either shareholders or employees of the Bone Health Technologies.
I genuinely look forward to learning about future, non-biased researchers coming up with similar conclusions about this product.
References
- https://www.bonehealthtech.com
- Bilek LD, et al. FRI681 OsteoboostTmIs Effective in Preserving Bone Strength and Density of the Spine in Women With Low Bone Mass. Journal of the Endocrine Society 2023 Oct-Nov; 7(1).